
Let us consider first the atmospheric gases. Table 1.1 illustrates the average gaseous composition of dry air below 25km. Although traces of atmospheric gases have been detected well out into space, 99% of the mass of the atmosphere lies below about 25 to 30km altitude, whilst 50% is concentrated in the lowest 5km (less than the height of Mount Everest).
Table 1.1. Average composition of the atmosphere below 25km
| Component | Chemical Abbreviation | Volume % (dry air) |
| Nitrogen | N2 | 78.08 |
| Oxygen | O2 | 20.98 |
| Argon | Ar | 0.93 |
| Carbon dioxide | CO2 | 0.035 |
| Neon | Ne | 0.0018 |
| Helium | He | 0.0005 |
| Hydrogen | H | 0.00006 |
| Krypton | Kr | 0.0011 |
| Xenon | Xe | 0.00009 |
| Methane | CH4 | 0.0017 |
| Ozone | O3 | 0.00006 |
Strictly speaking, the concentration of ozone in the atmosphere is variable.
Inert gases.
This gaseous mixture remains remarkably uniform in composition, and is the result of efficient biogeochemical recycling processes and turbulent mixing in the atmosphere. The two most abundant gases are nitrogen (78% by volume) and oxygen (21% by volume), and together they make up over 99% of the lower atmosphere. There is no evidence that the relative levels of these two gases are changing significantly over time (Kemp, 1994).
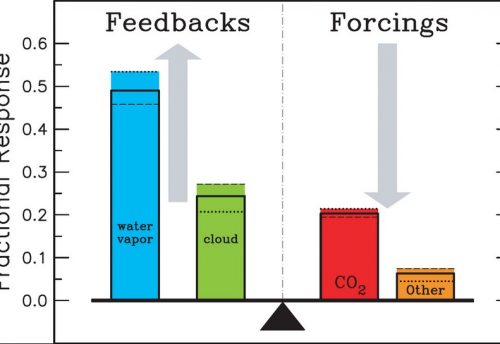
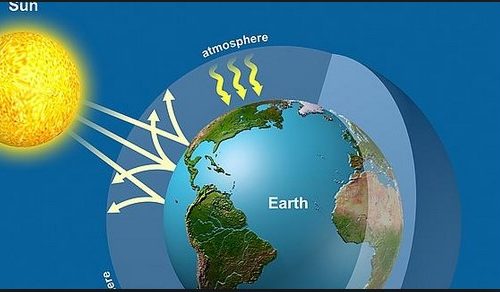
Despite their relative scarcity, the so-called greenhouse gases play an important role in the regulation of the atmosphere’s energy budget.

Leave a Reply